When warfarin works well, it can be lifesaving. When the dose is even slightly off, it can become one of the most unpredictable medicines in modern care. Overdosing exposes patients to severe bleeding. Underdosing leaves them vulnerable to blood clots that can cause strokes, pulmonary embolism or heart complications. Researchers at the National University Health System have now developed a small data artificial intelligence tool that may help clinicians navigate this delicate balance with unprecedented precision.
A new study led by Tiffany Gan Rui Xuan and colleagues, published in Bioengineering and Translational Medicine, introduces an AI platform called CURATE.AI that can optimise warfarin dosing using only a handful of personalised data points. Their paper titled AI assisted warfarin dose optimisation with CURATE.AI for clinical impact Retrospective data analysis explores how this methodology compares with conventional physician guided dosing. The findings hint at a future where affordable drugs like warfarin remain clinically powerful but with the safety and accuracy of personalised medicine.
A fragile drug with a global footprint
Warfarin has been a central part of anticoagulation therapy for more than sixty years. It is prescribed to prevent blood clots in patients with atrial fibrillation, deep vein thrombosis, pulmonary embolism or mechanical heart valves. Its popularity stems from its low cost and well-characterised safety profile. Millions of people in low and middle-income countries rely on it every day.
Despite its reliability on paper, warfarin acts almost like a moving target inside the human body. Its effect is measured by the international normalised ratio or INR. For most patients, the therapeutic range sits between 2.0 and 3.0. Levels below this range increase the risk of clot formation. Levels above it can lead to dangerous internal bleeding. Maintaining stability within this narrow therapeutic window is notoriously difficult.
Diet, genetics, illness, alcohol intake and drug interactions can influence how an individual metabolises warfarin. Physicians often adjust doses using experience and judgement rather than precise prediction. The result is frequent dose changes, repeated INR testing and a constant risk of oscillating outside the therapeutic range. These fluctuations contribute to increased hospital admissions, higher treatment costs, and a reduced quality of life for patients.
Newer anticoagulants are available, but they are expensive and unsuitable for individuals with mechanical heart valves or antiphospholipid syndrome. For a significant portion of the global population, warfarin remains indispensable; therefore, improving dose accuracy has become a priority in clinical pharmacology.
Where current dosing models fall short
Attempts have been made to refine warfarin management through dosing algorithms, pharmacokinetic and pharmacodynamic modelling, and even genetic testing. Many of these tools are impressive in theory but cumbersome in practice. They often require large datasets, population-level parameter estimates, or rapid genotyping capabilities that are not available in all clinical settings.
Studies referenced in the research report suggest that these models may be inaccurate when individual patient variability is high. Each person can respond differently to the same warfarin dose, and these responses can change over time. A personalised system that evolves with the patient has been difficult to achieve without excessive data requirements.
The study by Gan and her coauthors highlights an important gap. Most existing algorithms rely on big data approaches or long training periods, but still cannot fully account for real-time changes in a patient’s physiology. The limitations of these tools underscore the need for a method that can adjust dynamically using only the information available during routine care.
A personalised curve built from small data
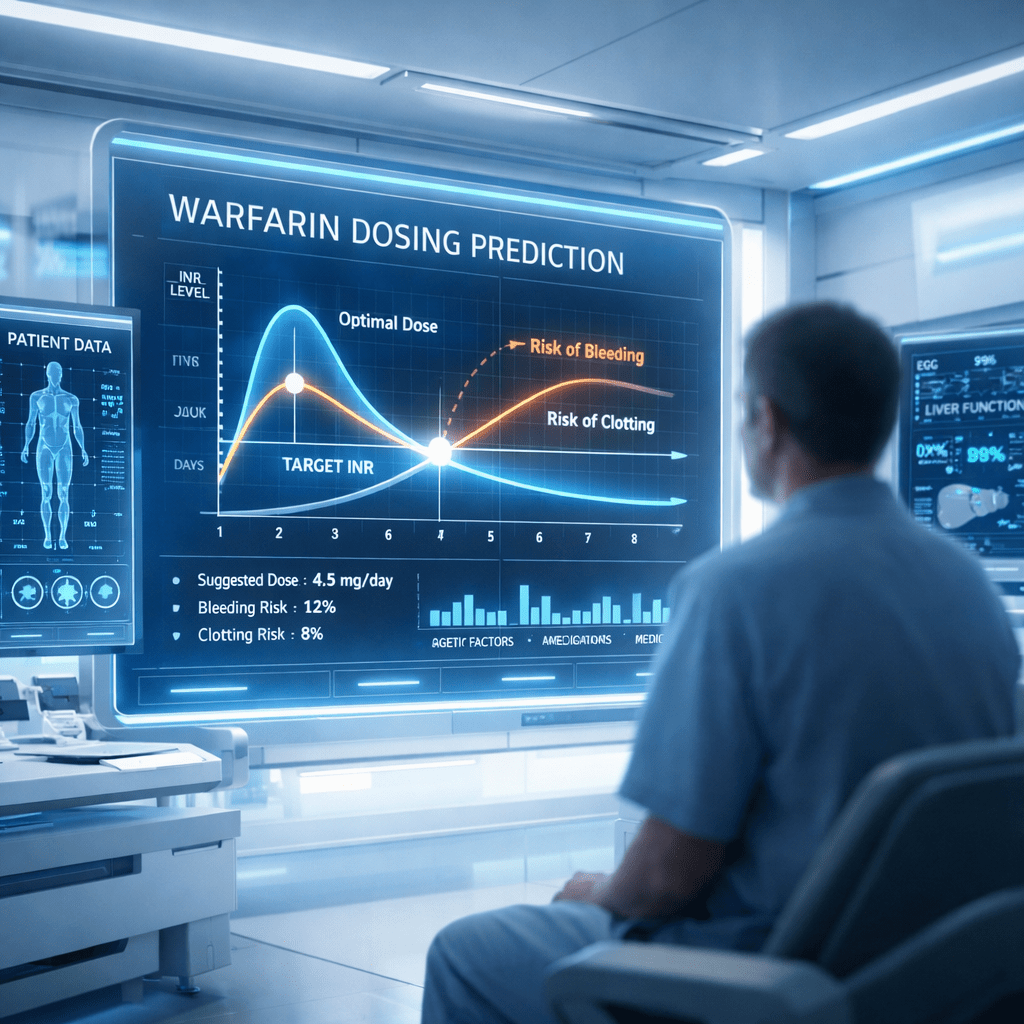
CURATE.AI takes a different approach. Instead of mining large datasets or predicting outcomes from detailed molecular models, it creates a personalised response profile for each patient. Using only two or three pairs of dose and INR response readings, the system constructs a mathematical curve that represents how the individual is responding to warfarin at that moment.
This personalised profile can be quadratic or linear depending on the data inputs. It is then used to predict how future doses might influence the patient’s INR. As more INR readings are collected, the AI updates this curve, enabling the profile to evolve in response to the patient’s condition. This makes the method robust against day-to-day fluctuations, which are common during warfarin therapy.
The authors note that CURATE.AI focuses entirely on phenotypic outputs rather than trying to model internal drug metabolism. In other words, the AI examines only what the body actually does in response to the drug, rather than how it might be expected to behave. This bypasses the complexities of genetics, diet, and drug interactions, yet still captures their combined influence. It also aligns with an emerging trend in precision medicine where dynamic patient-specific feedback informs treatment decisions.
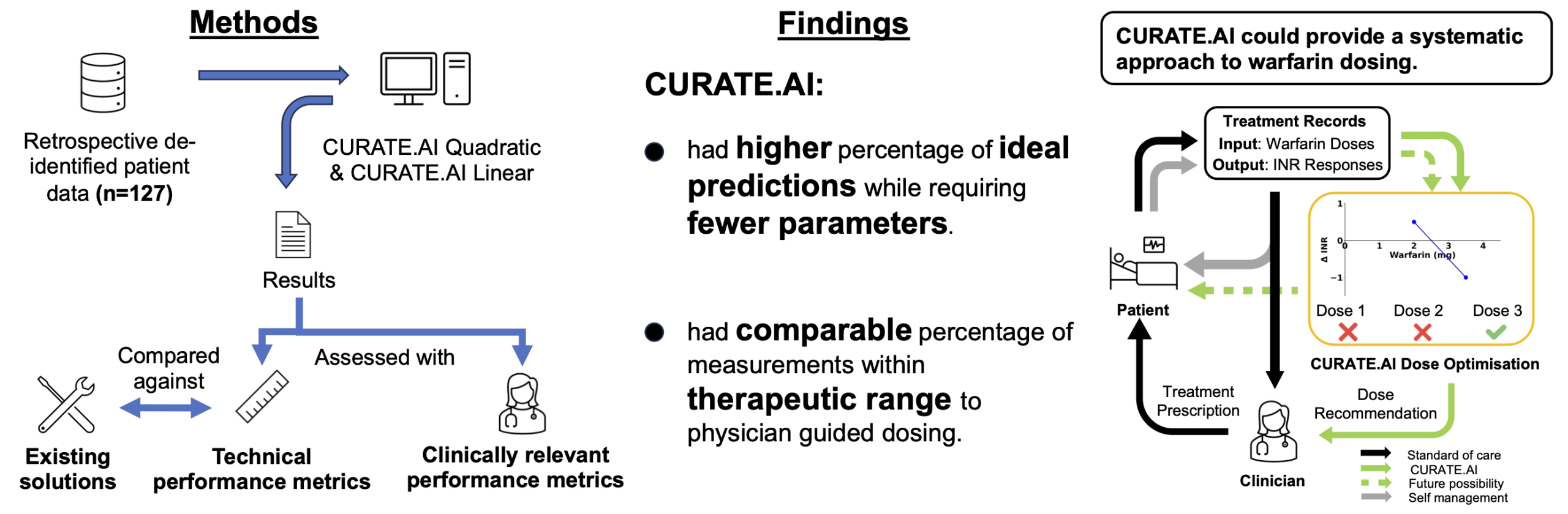
How CURATE.AI performed against existing dosing strategies
To evaluate the platform, the team analysed data from 127 patients treated at the National University Hospital in Singapore. Each patient had dose and INR records collected during routine care. The AI was tested retrospectively to predict INR values and identify the optimal dose that would maintain the therapeutic range.
Across more than 1,700 prediction events, CURATE.AI achieved ideal prediction accuracy in approximately sixty percent of cases, using the twenty percent error threshold common in the field. This performance was better than several established dose-response and PKPD models cited in the paper. The linear version of the system showed slightly better precision than the quadratic version, although both produced comparable levels of clinical relevance.
Crucially, the AI demonstrated negligible underprediction bias. Underprediction occurs when the system estimates an INR lower than the true value, which could prompt a clinician to increase the dose. For a drug like warfarin, this is a significant safety concern because it may lead to overdosing. The small bias observed in this study suggests that CURATE.AI may lower the chance of pushing patients into dangerous INR territory.
The researchers also calculated a modelled potential time in the therapeutic range. This metric estimates how much time a patient might spend within their target INR if dosing decisions were based on the AI. The results were comparable to those of physician-guided dosing, indicating that CURATE.AI could offer systematic and consistent support without compromising clinical effectiveness.
As a clinician, I have seen how a drug that saves lives can also place patients at risk when the margin for error is so narrow. This work is about respecting that fragility using artificial intelligence not to replace clinical judgement, but to support safer, more personalised decision-making in everyday care.
-Tiffany Gan Rui Xuan
What this could mean for patient care
Personalising drug dosing in real time is a challenge across many therapeutic areas. The small data approach behind CURATE.AI provides a practical alternative to large data and genetics-based models. It requires no specialised equipment and relies solely on measurements already collected during standard warfarin therapy.
For patients, this could translate to fewer dose adjustments, fewer clinic visits, and a lower risk of complications. For healthcare systems, it represents a way to integrate artificial intelligence into daily workflow without disrupting established treatment pathways. It may also help maintain long-term warfarin use in regions where newer anticoagulants are unaffordable or inappropriate.
The study does have limitations. INR measurements in the dataset were irregular and the retrospective design prevented prospective dose selection. The model also assumes a standard INR target of 2.0 to 3.0, although some patients may require alternative ranges. These constraints highlight the need for prospective clinical trials that test the AI in real-time decision-making.
A step towards clinically relevant artificial intelligence
One notable aspect of the study is its emphasis on both technical accuracy and clinical relevance. The authors propose new evaluation metrics, such as the INR clinical prediction error, which provides a more meaningful measure of safety compared with standard statistical error measures. This reflects a broader shift in the development of medical AI where clinical significance must be prioritised alongside algorithmic performance.
The research reveals how artificial intelligence can enhance established therapies without replacing clinical judgement. It suggests that the future of treatment optimisation may lie in tools that amplify the expertise of clinicians rather than bypass it. This blends human decision-making with algorithmic precision in a manner that supports safer and more consistent care.
Reference
Gan, T. R. X., Tan, L. W. J., Egermark, M., Truong, A. T. L., Kumar, K., Tan, S. B., Tang, S., Blasiak, A., Goh, B. C., Ngiam, K. Y., & Ho, D. (2025). AI assisted warfarin dose optimisation with CURATE.AI for clinical impact Retrospective data analysis. Bioengineering and Translational Medicine, 10(3), e10757.https://doi.org/10.1002/btm2.10757