In a world where our immune system is both protector and potential enemy, three scientists have been awarded the 2025 Nobel Prize in Physiology or Medicine for uncovering how this powerful force stays under control.
Mary E. Brunkow of the Institute for Systems Biology in Seattle, Fred Ramsdell of Sonoma Biotherapeutics in San Francisco, and Shimon Sakaguchi of Osaka University in Japan, were recognised “for their discoveries concerning peripheral immune tolerance.”
Their groundbreaking work revealed the existence of regulatory T cells, the immune system’s own peacekeepers, and the crucial role of the FOXP3 gene in maintaining balance. Together, their findings have reshaped modern immunology, paving the way for innovative treatments for autoimmune diseases, cancer, and organ transplantation.
When the body turns on itself
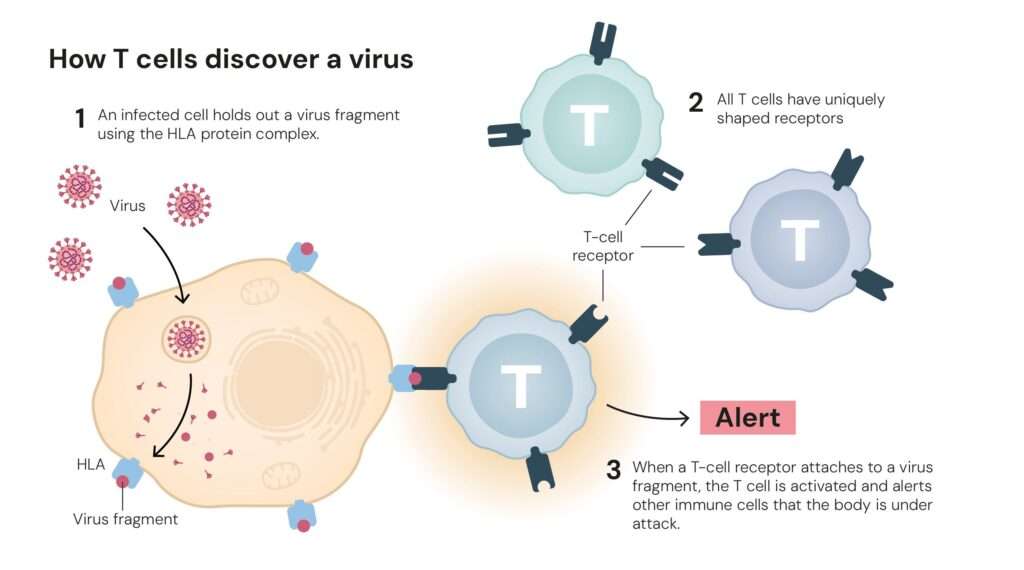
Every day, the immune system defends us against an army of invaders such as viruses, bacteria, and rogue cells that threaten our survival. But this defence must be exquisitely balanced. If immune cells misfire, they can attack the very tissues they are meant to protect, leading to conditions like type 1 diabetes, lupus, and multiple sclerosis.
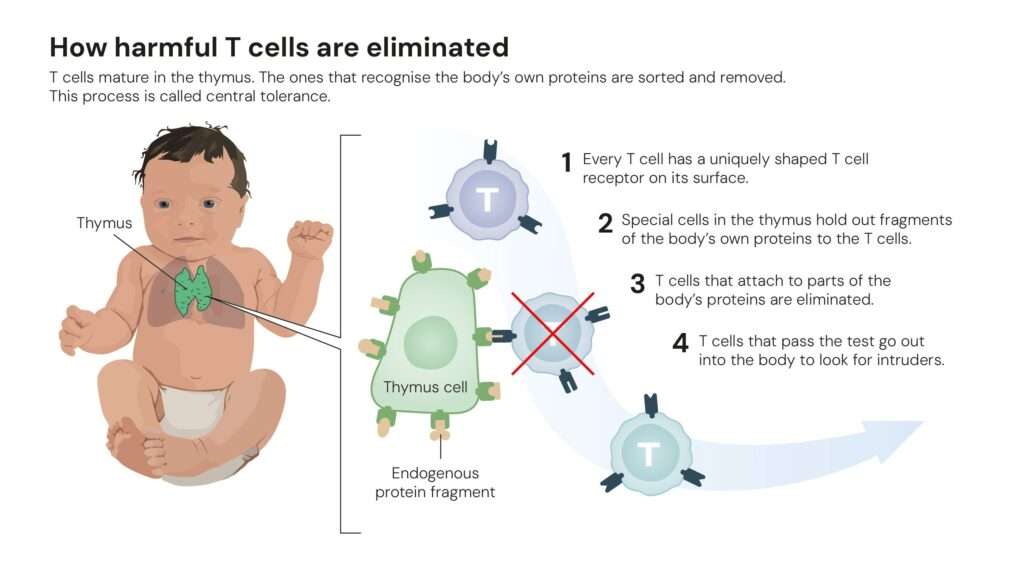
Until the 1990s, scientists believed the immune system relied mainly on a process called central tolerance, a rigorous screening in the thymus where self-reactive T cells were eliminated. Yet, this explanation left a puzzle: why did autoimmune reactions still occur even when this mechanism worked correctly?
The answer, it turned out, lay not in the thymus but in the body’s periphery, a discovery that changed the course of immunology.
Sakaguchi’s insight: Finding the immune system’s ‘security guards’
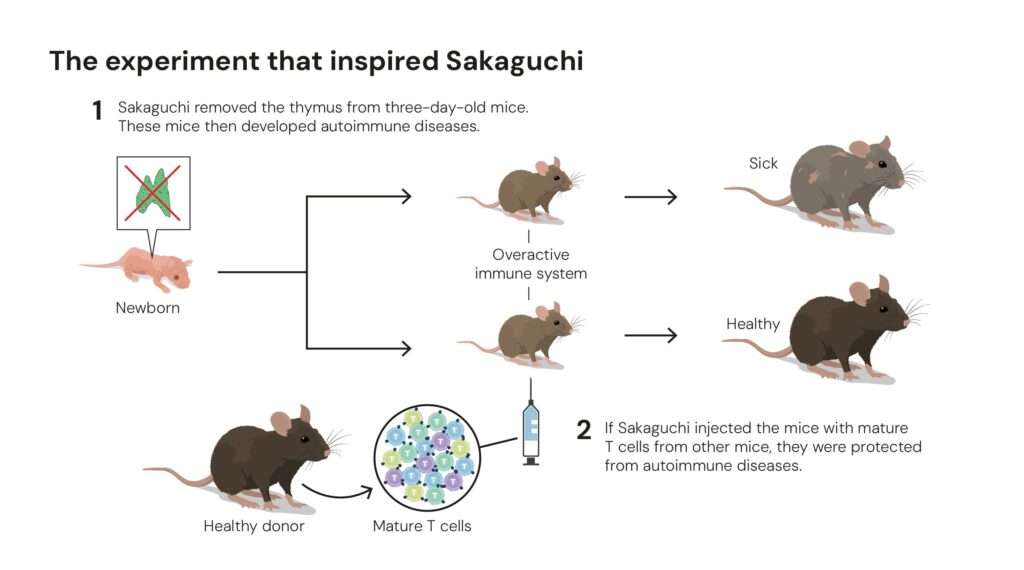
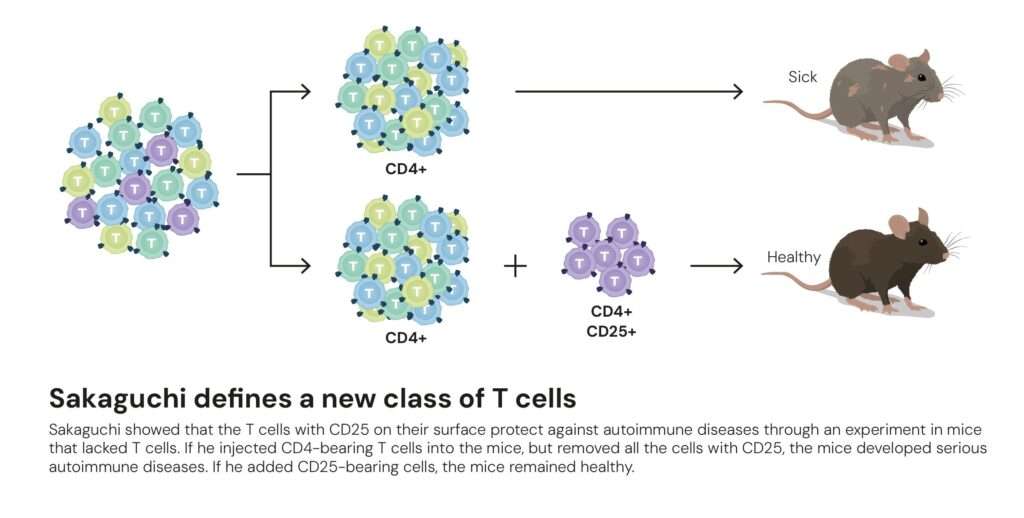
In the early 1980s, Shimon Sakaguchi, then a researcher at Japan’s Aichi Cancer Center Research Institute, conducted a simple but revolutionary experiment. He noticed that when the thymus was removed from newborn mice a few days after birth, their immune systems went berserk, attacking multiple organs.
When Sakaguchi reintroduced specific T cells into these mice, the autoimmune damage stopped. Something within those cells acted as a brake, a natural regulator of immune overactivity.
By 1995, after years of painstaking research, Sakaguchi identified this mysterious subset of cells. They bore two surface proteins, CD4 and CD25, and had a unique ability to suppress other T cells. He named them regulatory T cells, or Tregs, and published the findings in The Journal of Immunology.
At first, the scientific community was sceptical. But the missing pieces soon arrived from across the Pacific.
The scurfy mice that changed everything
Around the same time in the United States, Mary Brunkow and Fred Ramsdell were studying a peculiar strain of mice known as scurfy mice. These animals, first observed in the 1940s at Oak Ridge National Laboratory, were plagued by severe autoimmune symptoms such as inflamed skin, enlarged organs, and early death.
Working at Celltech Chiroscience in Bothell, Washington, Brunkow and Ramsdell set out to find the genetic cause of this immune chaos. In the 1990s, before modern sequencing technologies, this was a monumental task, searching for a mutation among 170 million base pairs on the mouse X chromosome.
After years of meticulous mapping, they struck gold. The mutation lay in a previously unknown gene belonging to the forkhead box family. They named it Foxp3.
In 2001, their landmark paper in Nature Genetics linked Foxp3 mutations in mice to a fatal immune disorder. Around the same time, they identified similar mutations in boys suffering from IPEX syndrome, a rare, life-threatening autoimmune disease. This confirmed that FOXP3 was not just crucial in mice, but essential for immune regulation in humans.
Connecting the dots between FOXP3 and the regulatory T cells
The discoveries by Brunkow, Ramsdell, and Sakaguchi converged rapidly. In 2003, Sakaguchi and colleagues published in Science that FOXP3 was the master gene controlling the development of regulatory T cells.
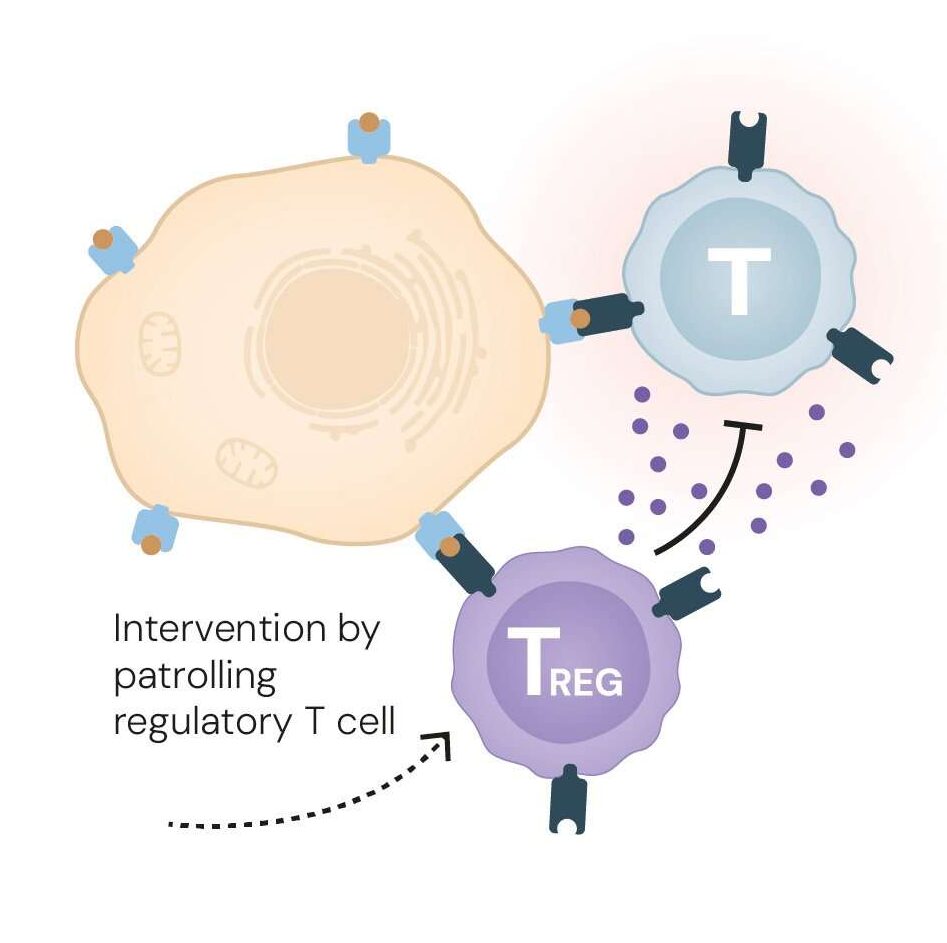
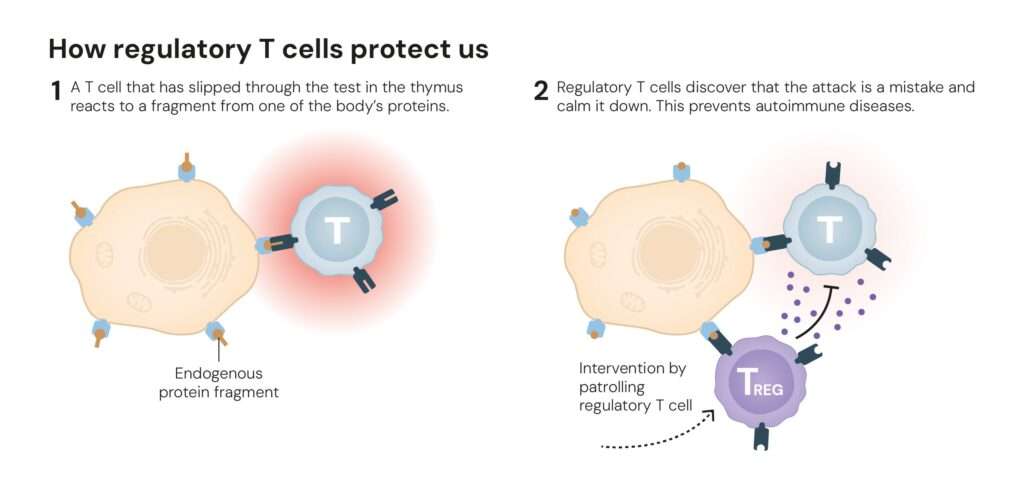
This breakthrough explained how the immune system avoids self-destruction. The FOXP3 protein acts like a molecular switch, programming certain T cells to become suppressors rather than attackers. These Tregs patrol the immune landscape, preventing overreactions and shutting down inflammation once infections are cleared, a process known as peripheral immune tolerance.
Without FOXP3 or functional Tregs, the immune system becomes anarchic, attacking healthy tissues as if they were foreign invaders.
Reprogramming the immune system
The trio’s discoveries have transformed medicine’s approach to immune-related diseases.
In autoimmune disorders, researchers are now exploring ways to boost Treg activity. Early clinical trials use interleukin-2 (IL-2), a molecule that encourages Treg growth, to calm conditions like lupus and type 1 diabetes. Other trials are testing Treg-based cell therapies, where patient-derived Tregs are expanded in the lab and reinfused to restore immune balance.
In organ transplantation, the same principle is being used to prevent rejection. Scientists are experimenting with engineered Tregs carrying ‘address labels’, antibodies on their surface that guide them to specific organs, such as a transplanted liver or kidney, to protect against immune attack.
Conversely, in cancer, the goal is the opposite, to weaken Tregs that shield tumours from immune destruction. Tumours often attract Tregs as a defence mechanism, effectively creating an “immune cloak.” By dismantling this protection, researchers hope to unleash the body’s own T cells against the cancer.
These therapies, still in early stages, are among the most promising applications of immunology today.
A new era of immune medicine
Today, the legacy of these discoveries extends far beyond the lab. Immunotherapy, once focused mainly on activating the immune system to fight cancer, is now expanding to suppress harmful reactions or restore balance.
Pharmaceutical companies and biotech firms are investing heavily in Treg-based treatments, with the hope of achieving what decades of immunosuppressants could not: precise, personalised immune modulation without side effects.
At the same time, understanding FOXP3’s regulation opens the door to genetic and epigenetic therapies, where reprogramming T cell identity could treat conditions from allergies to inflammatory bowel disease.
In the words of the Nobel Committee, Brunkow, Ramsdell, and Sakaguchi have “provided fundamental knowledge of how the immune system is regulated and kept in check.”
Their discoveries illuminate one of biology’s most elegant paradoxes — that protection requires restraint. By understanding how the immune system knows when to stop fighting, they have unlocked new frontiers in medicine that promise to protect millions from both disease and the immune system’s own fury.
Profiles of the laureates
Mary E. Brunkow (b. 1961)
PhD, Princeton University, USA. Senior Programme Manager at the Institute for Systems Biology, Seattle. Brunkow’s genetic detective work led to the discovery of the FOXP3 gene, unlocking a molecular key to immune regulation.
Fred Ramsdell (b. 1960)
PhD (1987), University of California, Los Angeles, USA. Scientific Advisor at Sonoma Biotherapeutics, San Francisco. Ramsdell’s leadership in linking FOXP3 to both scurfy mice and IPEX syndrome cemented the gene’s central role in immune homeostasis.
Shimon Sakaguchi (b. 1951)
MD (1976) and PhD (1983), Kyoto University, Japan. Distinguished Professor at the Immunology Frontier Research Center, Osaka University. Sakaguchi’s discovery of regulatory T cells laid the conceptual groundwork for immune tolerance therapies now being tested worldwide.

Key publications that shaped modern immunology
- Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor a-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995:155:1151-1164.
- Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, Wilkinson JE, Galas D, Ziegler SF, Ramsdell F. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001:27:68 73.
- Wildin RS, Ramsdell F, Peake J, Faravelli F, Casanova JL, Buist N, Levy-Lahad E, Mazzella M, Goulet O, Perroni L, Bricarelli FD, Byrne G, McEuen M, Proll S, Appleby M, Brunkow M. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet. 2001:27:18-20.
- Benne; CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, Kelly TE, Saulsbury FT, Chance PF, Ochs HD. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001:27:20-21.
- Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003:299:1057-1061.
These papers collectively redefined how scientists understand immune tolerance, autoimmunity, and immune therapy design.
References
Sakaguchi, S., Sakaguchi, N., Asano, M., Itoh, M., & Toda, M. (1995). Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. Journal of immunology (Baltimore, Md.: 1950), 155(3), 1151-1164. https://pubmed.ncbi.nlm.nih.gov/7636184/
Brunkow, M. E., Jeffery, E. W., Hjerrild, K. A., Paeper, B., Clark, L. B., Yasayko, S. A., … & Ramsdell, F. (2001). Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nature genetics, 27(1), 68-73. https://doi.org/10.1038/83784
Hori, S., Nomura, T., & Sakaguchi, S. (2003). Control of regulatory T cell development by the transcription factor Foxp3. Science, 299(5609), 1057-1061. https://doi/10.1126/science.1079490
Press release. NobelPrize.org. Nobel Prize Outreach 2025. Mon. 6 Oct 2025. https://www.nobelprize.org/prizes/medicine/2025/press-release/