Every breath you take may carry more than just oxygen. Invisible to the naked eye, tiny fungal spores drift in the air all around us. Most pass through unnoticed, causing no harm. But among them lurks Aspergillus fumigatus, a dangerous mould that can trigger severe, often fatal infections in people with weakened immune systems. The situation is becoming more urgent: resistance to antifungal medicines is rising, and the arsenal of available drugs is worryingly small.
In a new study published in Angewandte Chemie International Edition, first author Ajit Kumar Bishoyi and colleagues at Utrecht University have revealed how a natural immune peptide may hold the key to fighting these fungal superbugs. Using an advanced spectroscopic technique called solid-state nuclear magnetic resonance (ssNMR), the team uncovered exactly how this peptide attacks the fungus’s defences. The discovery could help design a new generation of antifungal drugs at a time when they are desperately needed.
The mounting threat of antifungal resistance
Fungal infections have long been overshadowed by the global crisis of antibiotic resistance. Yet, as health authorities warn, antifungal resistance is growing at an alarming pace. Aspergillus fumigatus, a common mould found in soil, plants and decaying matter, is at the forefront of this battle. In healthy individuals, inhaled spores are quickly neutralised by the immune system. But for cancer patients, transplant recipients, or people with chronic lung conditions, they can be lethal.
The danger has been amplified by the overuse of azole fungicides in agriculture. These chemicals, used to protect crops, are chemically similar to the drugs doctors prescribe for fungal infections. This dual exposure has driven the evolution of resistant strains outside hospitals, which can then infect humans. Once an azole-resistant strain takes hold, treatment options shrink dramatically. Mortality rates for invasive aspergillosis can exceed 50 per cent.
The challenge is compounded by the fact that current antifungal drugs often target the fungal cell membrane or internal machinery. But before they can reach those targets, the drugs must pass through the fungus’s cell wall – a formidable barrier designed to keep threats out. Understanding how to breach this wall is critical for developing new treatments.
I work on the BAM complex (which represents a central target for antibiotic development), with a particular emphasis on investigating its structural conformation and dynamics in the native bacterial membrane.
-Ajit Kumar Bishoyi
A promising peptide from an unexpected source
The research team turned their attention to cathelicidin-2 (CATH-2), a host defence peptide derived from chickens. Cathelicidins are small proteins produced naturally by many animals, including humans, as part of their immune response. They have a broad spectrum of activity against bacteria, viruses and fungi.
Earlier studies had shown that CATH-2 could inhibit azole-resistant A. fumigatus. However, its mode of action was unclear. Was it simply punching holes in the membrane, or did it have more subtle effects on the fungal structure?
To answer this, Bishoyi and his colleagues used solid-state NMR, a technique that works like a molecular MRI, mapping the structure and chemical environment of molecules inside complex materials. Crucially, they applied it directly to intact fungal mycelia, avoiding the limitations of studying isolated cell wall components.
Cracking the fungal armour
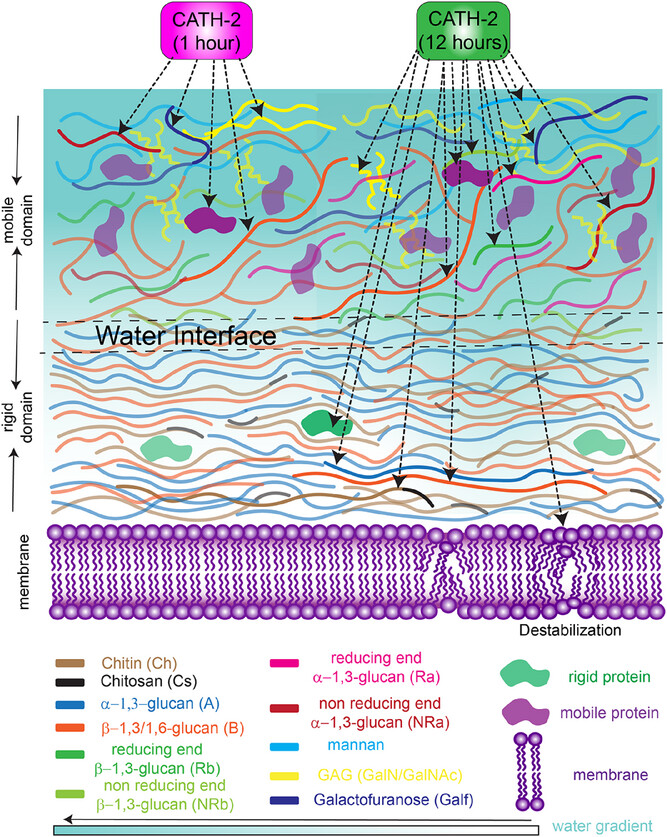
The cell wall of A. fumigatus is a multilayered structure. The rigid domain forms the tough outer shell, composed mainly of α-1,3-glucan, β-1,3-glucan and chitin. Inside lies the mobile domain, a softer, more flexible layer rich in galactomannan, galactosaminogalactan (GAG), proteins and lipids.
The Utrecht team discovered that CATH-2 first attacks the mobile domain. Within just one hour of exposure, ssNMR revealed a dramatic loss of signals from certain amino acids and polysaccharides, including galactosamine, N-acetyl galactosamine and specific forms of glucan. GAG, in particular, is a critical factor in the fungus’s ability to cause disease. Disrupting it could weaken the pathogen’s grip on host tissues.
Over longer exposures, the effects spread to the rigid domain. After 12 hours, the peptide potentially penetrated and increased the water permeability of the fungal wall. This change in hydration may be key to destabilising the fungus’s protective barrier.
Peering into the microscopic battlefield
One of the most striking findings came from analysing how the peptide altered water accessibility in the cell wall. Normally, only around 13 per cent of the rigid wall’s polysaccharides are accessible to water. After prolonged CATH-2 exposure, this figure jumped to around 30 per cent.
The ssNMR data suggested that the peptide was disrupting the intricate network of chemical bonds that hold the wall’s sugar polymers together. In doing so, it created new interfaces between hydrophilic (water-loving) and hydrophobic (water-repelling) regions.
Interestingly, the amino acids in the rigid domain – mostly hydrophobic and arranged in α-helices – remained buried deep within hydrophobic pockets, inaccessible to water. This supports the idea that CATH-2 works primarily by disturbing the polysaccharide scaffold rather than directly binding these proteins.
Why this matters now
The timing of this discovery is significant. Climate change, global trade, and increased medical interventions are all expanding the reach of fungal diseases. In 2022, the World Health Organization released its first list of priority fungal pathogens, placing A. fumigatus in the critical tier. The growing threat has prompted renewed interest in antifungal drug development, but progress has been slow.
Natural peptides like CATH-2 could accelerate this process. Because they act in multiple ways – targeting both flexible and rigid domains, increasing wall permeability, and potentially interfering with biosynthesis – it may be harder for fungi to evolve resistance.
Moreover, understanding the precise molecular interactions involved can guide the design of synthetic analogues. These could be optimized for human use, balancing potency with stability and safety.
The road ahead
While these results are promising, much work remains before CATH-2 or its derivatives can become clinical treatments. Future studies will need to test their efficacy in animal models of infection, determine optimal dosing strategies, and assess potential toxicity in human tissues.
The Utrecht team also suggests exploring labelled versions of the peptide to trace its exact binding sites within the fungal wall. Combining this with time-resolved NMR could reveal how the fungus responds at each stage of the attack, providing further insights for drug design.
Rethinking the fight against fungal infections
This study underscores the value of ssNMR (one of the structural biology techniques) in tackling pressing medical challenges. By showing exactly how a natural molecule dismantles a fungal pathogen’s defences, Bishoyi and colleagues have opened the door to new strategies against drug-resistant fungi.
In the broader context, it is a reminder that nature has been engaged in microbial warfare for millions of years. The molecules evolved by animals, plants and microbes could inspire tomorrow’s medicines – if we have the tools to decode them.
With antifungal resistance on the rise, the stakes are high. The lessons from CATH-2’s attack on A. fumigatus may one day help save the lives of patients facing otherwise untreatable infections.
Could nature hold the answer?
As drug-resistant fungal infections spread, the question is no longer whether we need new treatments, but how quickly we can develop them. The story of CATH-2 offers a hopeful glimpse into one possible future.
If researchers can translate these molecular insights into safe, effective drugs, we might one day have an arsenal capable of outmaneuvering even the toughest fungal foes. But doing so will require sustained investment, interdisciplinary collaboration, and the recognition that fungal disease is as urgent a threat as bacterial resistance.
Could the humble chicken be the unlikely ally that tips the balance in this fight?
Reference
Bishoyi, A. K., van Neer, J., Bahri, S., Lorenz, S., de Cock, H., & Baldus, M. (2025). Solid-state NMR reveals reorganisation of the Aspergillus fumigatus cell wall due to a host-defence peptide. Angewandte Chemie International Edition. Advance online publication. https://doi.org/10.1002/anie.202509012