When the Royal Swedish Academy of Sciences, 2025 announced the 2025 Nobel Prize in Chemistry, it did more than celebrate three exceptional scientists. It recognised a quiet revolution in molecular design, a shift from chemistry that builds individual molecules to chemistry that constructs vast, porous architectures capable of solving global challenges.
The laureates, Susumu Kitagawa, Richard Robson, and Omar M. Yaghi, were honoured “for the development of metal–organic frameworks”. These crystalline materials, known as metal– organic frameworks (MOF), are intricate molecular lattices that can trap, filter, or store specific molecules with extraordinary precision. In them, scientists have found ways to harvest water from desert air, capture carbon dioxide from power plants, and store hydrogen for a cleaner energy future.

The revolution in molecular architecture
For decades, chemists have been masters of creating discrete molecules, the single entities that define traditional chemistry. Yet the design of extended structures that stretch in two or three dimensions remained elusive. Predicting how atomic building blocks could assemble into stable, porous solids was one of chemistry’s greatest challenges.
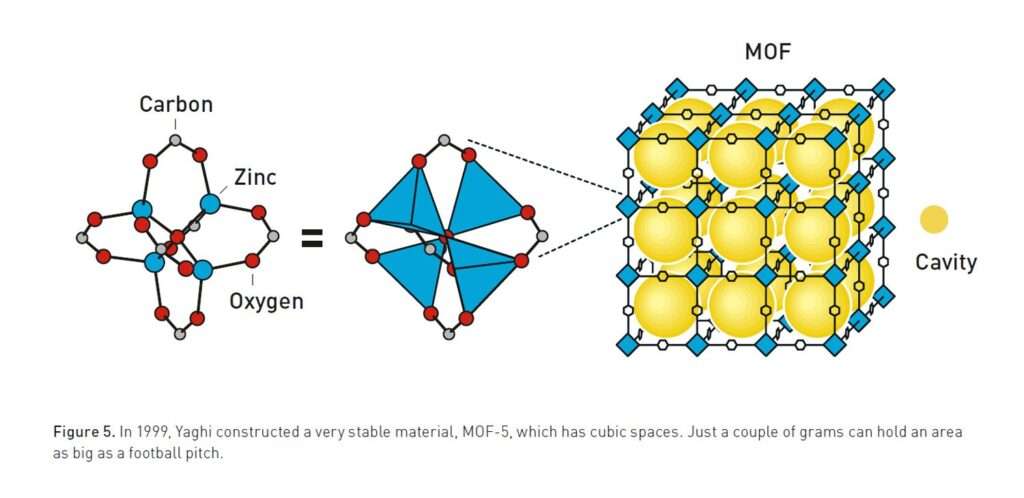
These structures combine metal ions with organic linkers to form vast, repeating lattices. Unlike dense materials such as diamond, MOFs are extraordinarily open. Their internal voids, sometimes making up more than 90 per cent of their volume, provide vast internal surface areas, where gases or liquids can be captured, stored, or transformed. A few grams of some MOFs can contain a surface area equal to a football pitch.
Richard Robson: The modeller who imagined new chemical spaces
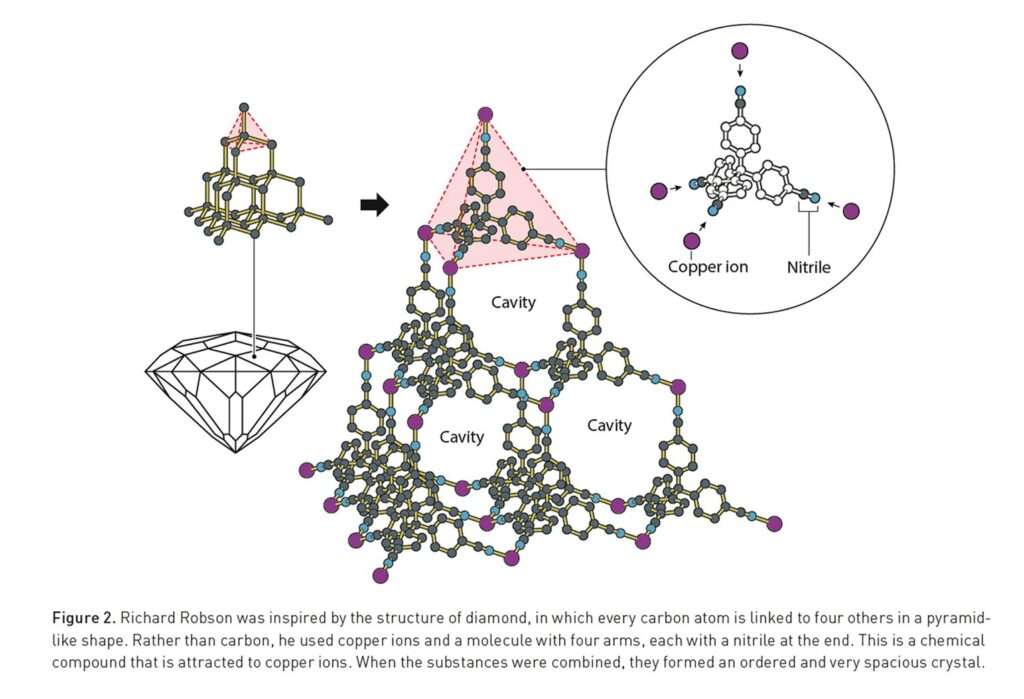
The story began in Melbourne in the 1970s, when Richard Robson, then a lecturer at the University of Melbourne, was preparing molecular models for his students. As he drilled holes in wooden spheres to represent atomic bonds, an idea struck him: the geometry of atoms (the directionality of their bonds) could be used as a blueprint to build larger, ordered frameworks.
More than a decade later, he tested his hypothesis. Using copper(I) ions as nodes and a tetranitrile molecule with four arms as linkers, Robson created a crystalline diamondoid structure, a framework that resembled the atomic geometry of diamond but was full of spacious cavities. When published in The Journal of the American Chemical Society in 1989, his discovery revealed that rationally designed coordination between metal ions and organic ligands could yield ordered, porous materials.
Robson’s frameworks demonstrated properties previously unimaginable. They could exchange ions, suggesting that substances could move freely in and out. They hinted at applications in catalysis and separations, functions later realised by the MOF field. At the time, however, many chemists dismissed these fragile crystals as laboratory curiosities. Yet for a few, they ignited a pioneering spirit. Robson had shown that chemistry could be engineered with geometry.
Susumu Kitagawa: Seeing usefulness in the useless
In Japan, another visionary was preparing to challenge conventional thinking. Susumu Kitagawa, now a professor at Kyoto University, was guided by a principle he learned from Nobel Laureate Hideki Yukawa: to find the usefulness of useless things. When he began exploring porous coordination compounds in the early 1990s, his goal was not immediate application but creative exploration.
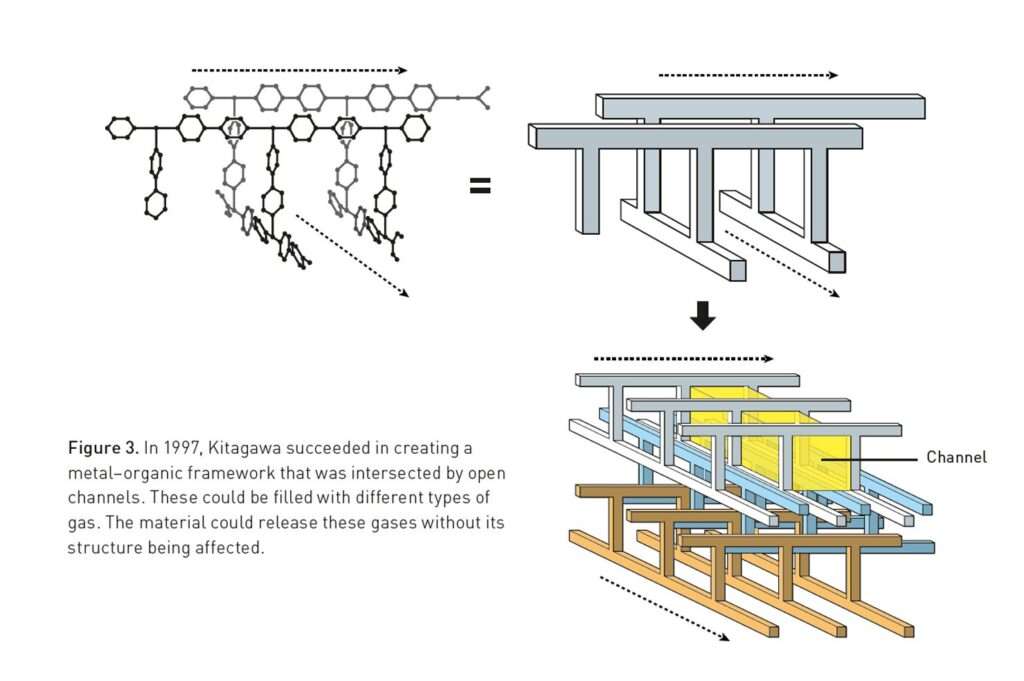
His early frameworks, built from copper ions and organic linkers, were unstable and lacked clear utility. Funding agencies rejected his proposals. Yet he persisted. By 1997, working with cobalt, nickel, and zinc ions, Kitagawa and his group at Kindai University created the first three-dimensional metal–organic frameworks with stable, open channels. These materials could adsorb and release gases such as methane and nitrogen without collapsing.
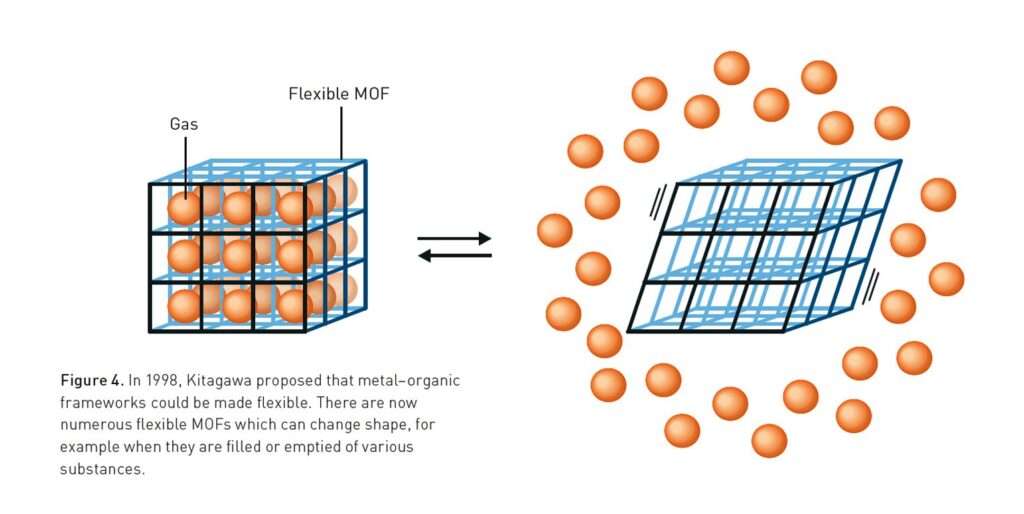
In 1998, Kitagawa went further. In a seminal paper in the Bulletin of the Chemical Society of Japan, he introduced the idea of flexible frameworks that could respond dynamically to external stimuli. Unlike rigid zeolites, MOFs could be soft and adaptive. This led to the concept of “soft porous crystals”, materials that behave like molecular lungs, inhaling and exhaling gases without structural breakdown.
Omar Yaghi: From Jordan to Berkeley, designing reticular chemistry
In a small library in Amman, Jordan, a ten-year-old Omar M. Yaghi once sneaked into a locked room filled with mysterious science books. Among them, he found diagrams of molecules that sparked a lifelong fascination with chemistry. Decades later, as a professor at the University of California, Berkeley, he would redefine how molecules are built.
After earning his PhD in 1990 at the University of Illinois Urbana-Champaign, Yaghi sought a more controlled way to design materials. Instead of random chemical reactions, he envisioned assembling atoms and molecules like Lego pieces. At Arizona State University in 1995, his group produced two-dimensional metal–organic frameworks using copper and cobalt ions connected by organic linkers. He coined the term “metal–organic framework”, defining a new class of crystalline, porous solids.
The turning point came in 1999 with the creation of MOF-5, a zinc-based structure held together by terephthalate linkers. Published in Nature, this framework displayed extraordinary stability and porosity, maintaining its structure even after being heated to 300°C. Two grams of MOF-5 had an internal surface area equal to a football pitch.
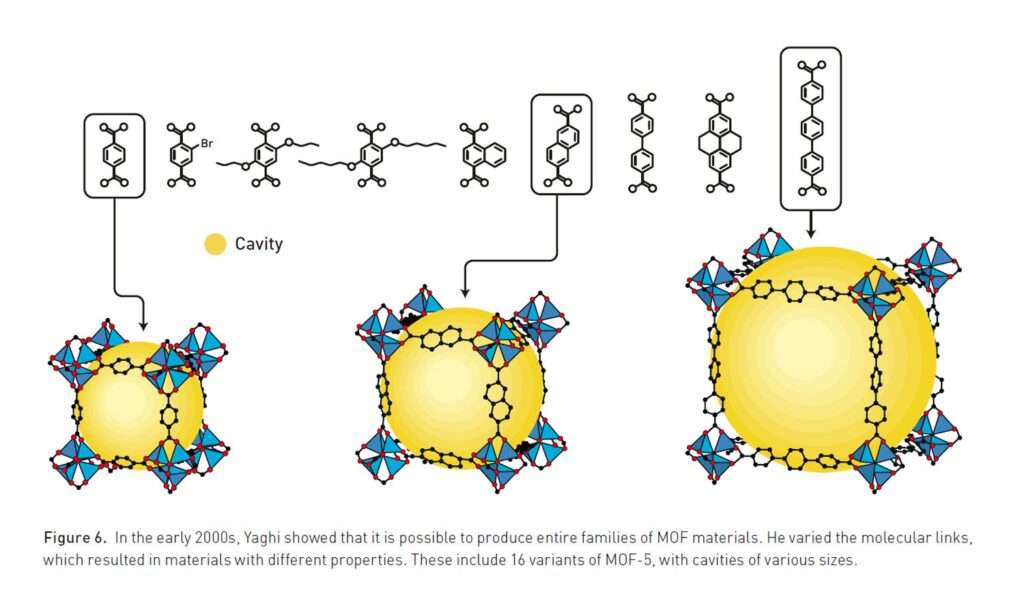
Yaghi’s innovations introduced reticular chemistry, the rational design and assembly of pre-defined molecular building blocks into predictable networks. This approach gave chemists unprecedented control over matter, enabling the creation of frameworks with tunable cavities and functionalities.
The science of flexible solids
One of the most profound ideas to emerge from Kitagawa’s and Yaghi’s research is that solids can be dynamic. Traditional materials like metals and ceramics are rigid and unresponsive. MOFs, in contrast, can breathe, flex, and reconfigure.
Kitagawa classified MOFs into three generations. The first collapsed when emptied of guest molecules. The second remained stable when gases were adsorbed or released. The third generation, the soft porous crystals, could change shape in response to external stimuli such as pressure or temperature. This redefined the boundaries of solid-state chemistry. In short, Robson provided the geometric foundation, Kitagawa demonstrated stability and flexibility, and Yaghi unified the field with conceptual clarity.
Their influence extends far beyond chemistry. MOFs have inspired innovations in materials science, physics, environmental engineering, and nanotechnology. These dynamic frameworks opened up possibilities for responsive materials that adapt to their environment. Such behaviour is crucial for advanced applications, from selective catalysis to smart gas sensors.
From laboratory to life: MOFs that shape the modern world
The collective work of Robson, Kitagawa, and Yaghi laid the foundation for a field that has since exploded in scale and ambition. Today, more than 100,000 MOFs have been synthesised, each with a unique structure and function.
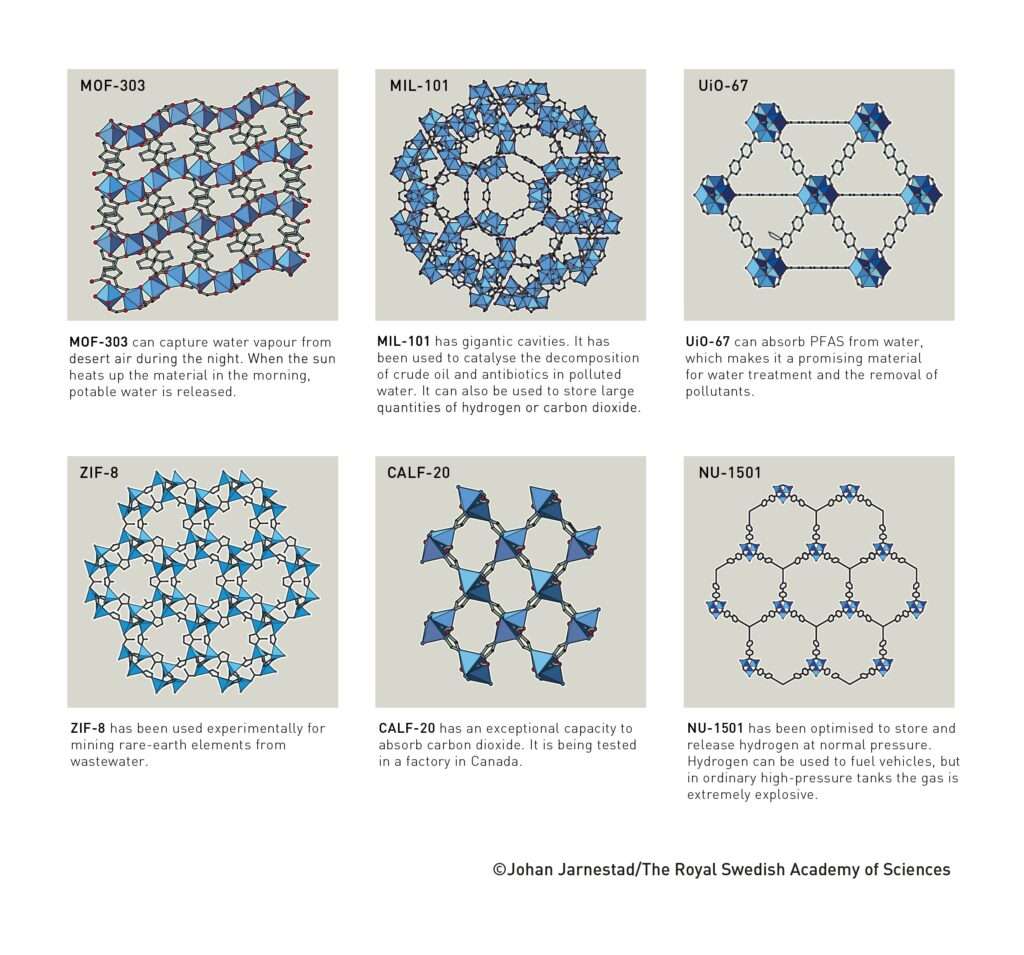
Some of these frameworks address the most pressing environmental challenges. MOF-303, developed in Yaghi’s laboratory, can harvest water vapour from desert air at night and release it as liquid water when warmed by the morning sun. CALF-20, another breakthrough, has shown exceptional carbon dioxide capture efficiency and is being tested in industrial settings in Canada.
Other frameworks such as MIL-101 and NU-1501 are designed for hydrogen storage, providing safe and efficient ways to store clean energy. UiO-67 can absorb per- and polyfluoroalkyl substances (PFAS) from contaminated water, while ZIF-8 is being tested for rare-earth element recovery from wastewater.
These examples reveal MOFs as multifunctional tools for sustainability. They can purify air and water, catalyse chemical reactions, store fuels, and even act as drug carriers in biomedical research.
Beyond the laboratory
Industrial collaborations have begun turning MOFs into market-ready technologies. Companies are exploring MOF-based systems for carbon capture, hydrogen fuel storage, and environmental remediation. In 2021, CALF-20 became one of the first MOFs tested at a commercial carbon capture plant. Its selective adsorption properties outperformed many existing sorbents, offering a path toward more efficient greenhouse gas mitigation.
In healthcare, MOFs are being studied for drug delivery and biochemical sensing, leveraging their tunable pore sizes and biocompatibility. Meanwhile, the electronics industry uses MOFs to trap and neutralise toxic gases in semiconductor manufacturing.
As machine learning and AI-driven material design enter the field, the next generation of frameworks is being created virtually before being synthesised in the lab. This computational design promises faster discovery and precise control over material behaviour.
The legacy of three visionaries
In 2025, the Nobel Committee described their achievement as creating “new rooms for chemistry”. The phrase captures not only the literal cavities within MOFs but also the intellectual space they opened for generations of researchers.
The potential of MOFs continues to expand. Their exceptionally high surface areas, often exceeding 7,000 m² per gram, and their tunable chemical properties make them ideal candidates for sustainable technologies. Researchers are exploring hybrid MOF composites, thin-film coatings, and even MOF glasses, extending their applications to electronics, energy storage, and environmental protection.
Through metal–organic frameworks, the 2025 Nobel Laureates have given humankind a powerful toolkit to address some of its most urgent challenges. Their discovery, as the Nobel Committee put it, has truly brought “the greatest benefit to humankind.”
Profiles of the laureates
Susumu Kitagawa, born 1951 in Kyoto, Japan. PhD 1979 from Kyoto University, Japan. Professor at Kyoto University, Japan.
Richard Robson, born 1937 in Glusburn, UK. PhD 1962 from University of Oxford, UK. Professor at University of Melbourne, Australia.
Omar M. Yaghi, Born 1965 in Amman, Jordan. PhD 1990 from University of Illinois Urbana-Champaign, USA. Professor at University of California, Berkeley, USA.
Prize Amount: 11 million Swedish kronor (~1.2 millon USD), to be shared equally between the laureates.
References
- Hoskins, B. F., & Robson, R. (1989). Infinite polymeric frameworks consisting of three dimensionally linked rod-like segments. Journal of the American Chemical Society, 111(15), 5962-5964. https://doi.org/10.1021/ja00197a079
- Kitagawa, S., & Kondo, M. (1998). Functional micropore chemistry of crystalline metal complex-assembled compounds. Bulletin of the Chemical Society of Japan, 71(8), 1739-1753. https://doi-org.tudelft.idm.oclc.org/10.1246/bcsj.71.1739
- Li, H., Eddaoudi, M., O’Keeffe, M., & Yaghi, O. M. (1999). Design and synthesis of an exceptionally stable and highly porous metal-organic framework. nature, 402(6759), 276-279. https://doi.org/10.1038/46248
- Royal Swedish Academy of Sciences. (2025). The Nobel Prize in Chemistry 2025 – Popular Science Background: They have created new rooms for chemistry. Retrieved from https://www.kva.se/en/pressroom/pressreleases/the-nobel-prize-in-chemistry-2025
- Royal Swedish Academy of Sciences. (2025). Nobel Prize in Chemistry 2025 – Scientific Background: Metal–Organic Frameworks. https://doi.org/10.6028/KVA.CHEM.2025.001