When we enjoy a steak or a lamb chop, we rarely think about what happens inside our bodies afterwards. Beyond digestion, our meals also nourish a hidden ecosystem of trillions of microbes living in the gut. These microbes are not passive. They actively process nutrients and produce chemicals that can help or harm our health.
One chemical attracting increasing scientific attention is trimethylamine N oxide (TMAO). High levels of TMAO in the blood are linked to cardiovascular disease, including heart attack and stroke. But where does TMAO come from, and why do some people produce more of it than others even when they eat the same food?
A recent study by Dr Wei Kai Wu and colleagues at National Taiwan University Hospital sheds new light on this question. Their research shows that the answer lies in gut microbes, specifically in a set of microbial genes known as gbu. These genes decide whether certain gut bacteria convert nutrients from red meat and other foods into trimethylamine (TMA), which the liver then turns into TMAO.
This discovery not only deepens our understanding of how diet and microbes interact but also opens the door to personalised nutrition strategies that could lower the risk of disease.
The red meat connection
Red meat, eggs, and dairy products all contain a nutrient called L carnitine. On its own, carnitine plays a vital role in energy metabolism by transporting fatty acids into cells where they can be used as fuel. The story changes when gut microbes become involved. Some microbes break down carnitine into trimethylamine (TMA), a gas with a strong fishy smell.
TMA does not remain in the gut but moves into the bloodstream and is carried to the liver, where enzymes convert it into TMAO. High levels of TMAO in the blood have been linked in many studies to cardiovascular problems. The compound may contribute to hardening of the arteries, greater blood clotting, and other processes that harm the heart and blood vessels.
The surprising part is that not everyone produces TMAO in the same way. Some people’s microbes convert a lot of carnitine into TMA, while others produce very little. Explaining this difference has been a long-standing scientific puzzle.
A genetic signature in gut microbes
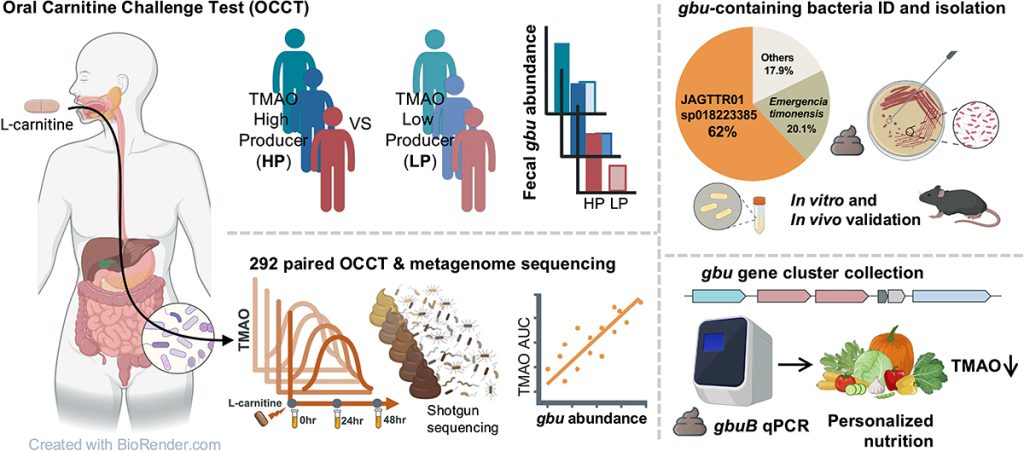
Dr Wu’s team focused on a particular group of microbial genes known as the gbu cluster, short for gamma butyrobetaine utilisation. These genes code for enzymes that allow bacteria to use a molecule called gamma butyrobetaine (γBB), a key step in breaking down carnitine into TMA.
By analysing stool samples from volunteers, the researchers discovered that people whose gut microbes carried the gbu genes were far more likely to produce TMAO after consuming L carnitine. In contrast, those without these genes in their microbiome showed little or no increase in blood TMAO.
The presence of the gbu gene cluster in gut bacteria therefore acts as a biomarker, a measurable indicator of whether someone is likely to convert dietary carnitine into potentially harmful TMAO.
A simple test to predict risk
To make their discovery useful in practice, the team created a simple stool DNA test that can detect the gbu genes. Much like a pregnancy test or a rapid COVID test, this tool could help predict health risks in a personalised way.
Imagine two people who both enjoy eating red meat. One carries gut bacteria with the gbu cluster while the other does not. After eating the same steak, the first person’s TMAO levels rise sharply, potentially increasing heart risk, while the second person’s levels remain low.
With this test, doctors could identify patients who are “high TMAO producers” and offer tailored dietary advice. Those at greater risk might limit their intake of red meat or try supplements and probiotics that encourage a healthier balance of gut microbes.
Precision nutrition in action
This discovery is part of a fast-growing field known as precision nutrition. Instead of offering one set of rules for everyone, precision nutrition takes into account a person’s genes, lifestyle, and microbiome to provide individual guidance. Dr Wu’s study gives one of the clearest examples so far of how the microbiome can decide whether a food is harmful or harmless.
The same diet may affect people differently, depending on the microbes they carry. By identifying these microbial genes, we can better understand who is at risk and who is not.
-Dr. Wei-Kai Wu
Implications beyond heart disease
Although the study focused on cardiovascular risk, the implications go much further. TMAO has also been linked to kidney disease, diabetes, and some cancers. If doctors can identify patients who are prone to high TMAO production, they may be able to step in earlier to prevent or manage several chronic conditions.
The discovery of the gbu pathway also opens up possibilities for new treatments. Researchers could, for example, design drugs or probiotics that block the activity of gbu genes and reduce TMAO production, without the need for strict dietary limits.
Taiwan at the forefront
The study was carried out at National Taiwan University Hospital (NTUH) and National Taiwan University (NTU), where Dr Wu leads the WKW Lab. His team specialises in gut microbiome research, using multi omics approaches that combine genomics, metabolomics, and other large scale analyses to capture a complete picture of microbial activity. This work highlights Taiwan’s growing role in microbiome science and its impact on human health. With advanced laboratory facilities and strong international collaborations, NTUH is emerging as a hub for microbiome based precision medicine.
Looking ahead
Science often moves forward in small steps, but occasionally a discovery marks a major leap. Identifying the gbu gene cluster as the key switch for TMAO production is one of those leaps. It links diet, gut microbes, and disease in a way that is both scientifically sound and practically useful.
The next stage will be to test this approach in larger populations, improve the diagnostic tools, and explore possible interventions. Could probiotics outcompete gbu positive bacteria and reduce TMAO levels? Could diets be personalised based on microbial testing? These are the questions now driving research in this field.
What this means for you
So what does this mean for the average person? In the future, health checks may not only measure blood pressure and cholesterol but also reveal a personal microbial profile. Instead of broad advice such as “eat less red meat,” doctors could one day say, “based on your gut microbes, you are more or less sensitive to carnitine intake.” This marks a shift from population based nutrition guidelines to truly individualised healthcare. And it all begins with the microbes in our gut and the powerful genes they carry.
Reference
Wu, W. K., Lo, Y. L., Chiu, J. Y., Hsu, C. L., Lo, I. H., Panyod, S., … & Wu, M. S. (2025). Gut microbes with the gbu genes determine TMAO production from L-carnitine intake and serve as a biomarker for precision nutrition. Gut Microbes, 17(1), 2446374. https://doi.org/10.1080/19490976.2024.2446374